Study design
The SENTIX trial was a prospective observational study that evaluated the safety of sentinel lymph node (SLN) biopsy as a less radical surgical approach for patients with early-stage cervical cancer.
The study aimed to determine whether SLN biopsy was non-inferior to systematic pelvic lymphadenectomy in terms of recurrence rates while reducing postoperative complications such as lower limb lymphedema.
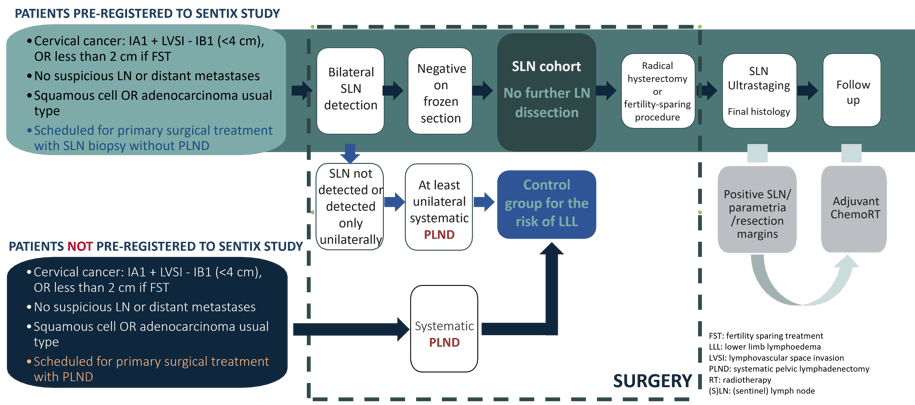
Primary endpoint
Recurrence rate (RR) at 24th month of follow-up
(not counting cervix recurrences after fertility-sparing procedures)
Reference recurrence rate: 7% (at the 24th month follow-up) in patients after systematic pelvic lymphadenectomy.
Margin of non-inferiority: 3%.
Additional endpoints
Disease-Free Survival
Pelvic Disease-Free Survival
Overall Survival
Prevalence of lower limb lymphedema
Quality of Life
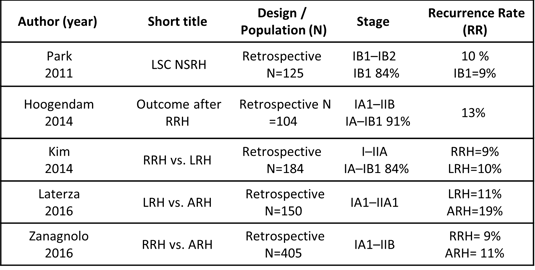
*Only few relevant examples shown.
Reference recurrence rate
Reference RR was based on historical cohorts of patients (IB1, no LN+ or low proportion of LN+)
27 manuscripts
Data on more than 8700 patients (approx. 100-1400/study)
RR in the range of 3-29%, median RR between 7%-13%
Sample size: 600
Non-inferiority margin at 24. months: 3%
Power: 90%
Significance: 0.05
Non-inferiority (P0) was determined using a one-sided Z-test.
Study protocol


