Less Invasive, Same Protection – The SENTIX Approach.
The SENTIX trial was an international clinical study focused on improving surgical treatment for women with early-stage cervical cancer. Traditionally, treatment includes the removal of many pelvic lymph nodes (systematic pelvic lymphadenectomy) to check for cancer spread. While effective, this approach can lead to significant post-surgical complications, including lymphedema (swelling of the legs due to lymph fluid buildup) and other side effects that impact quality of life.
The SENTIX trial aimed to determine whether sentinel lymph node (SLN) biopsy, a less invasive technique that targets only key lymph nodes, could provide the same level of cancer control while reducing complications. SLN biopsy has already been successfully used in breast and vulvar cancer, but its role in cervical cancer had not been fully established. The study was designed to assess whether SLN biopsy alone, combined with advanced pathological testing (ultrastaging), could safely replace full lymph node removal.
This was a large-scale, multicenter study, involving more than 800 patients from 47 centers across 18 countries, making it one of the most comprehensive trials in this field.
Main Findings:
1. Safe and Effective:
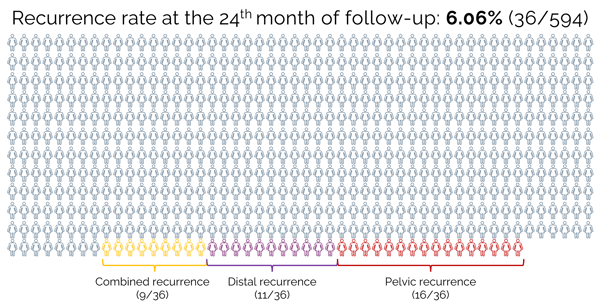
The study confirmed that removing a small number of sentinel lymph nodes does not worsen treatment outcomes compared to removing a larger number of pelvic lymph nodes.
2. Highly Accurate:
Careful processing of SLN by the pathologist significantly increases accuracy, as it allows the detection of small metastases that would otherwise go unnoticed.
3. Improving quality of life:
Removing only a small number of SLNs reduces postoperative complications compared to the removal of a larger number of lymph nodes.