SENTIX study
Survival of patients with early stages cervical cancer after sentinel lymph node (SLN) biopsy without systematic pelvic lymphadenectomy
SENTIX prospective, single-arm, non-inferiority, international trial
(CEEGOG CX-01; ENGOT-CX2)
833
Enrolled patients (SLN + control)
SENTIX trial has successfully met its primary endpoint, demonstrating that SLN biopsy is a safe and effective alternative to systematic pelvic lymphadenectomy(PLND).
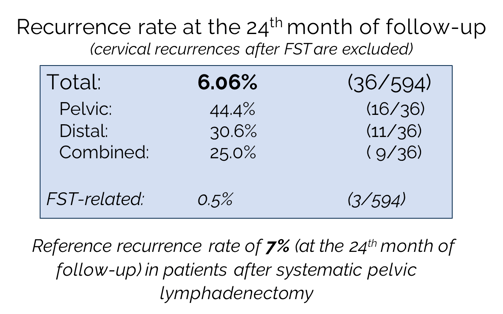
Recurrence rate (RR) at 24 months: 6.06%, confirming non-inferiority to the reference 7% RR observed after systematic pelvic lymphadenectomy.
Excellent 2-year DFS 93.3% and OS 97.9% after SLN surgery, comparable to patients after PLND, reported in historical or recent prospective trials.
Recurrence rate (RR) at 24 months: 6.06%, confirming non-inferiority to the reference 7% RR observed after systematic pelvic lymphadenectomy.
Excellent 2-year DFS 93.3% and OS 97.9% after SLN surgery, comparable to patients after PLND, reported in historical or recent prospective trials.
594
ITT patients
36
Recurrences at 24 months
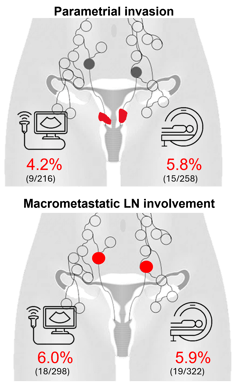
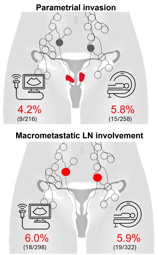
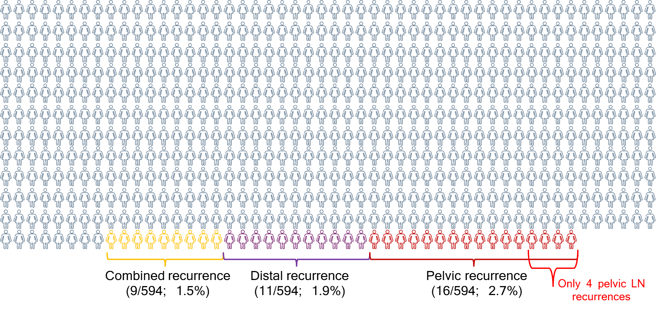
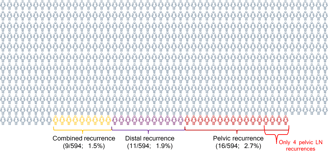
16 (44%) Pelvic
11 (31%) Distant
9 (25%) Combined (pelvic+ distant)
Study outcomes

Survival
outcomes
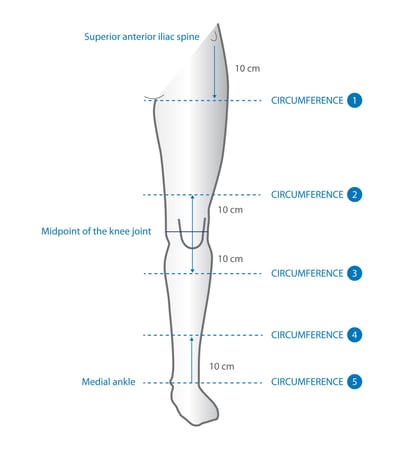
Lower limb
lymphoedema
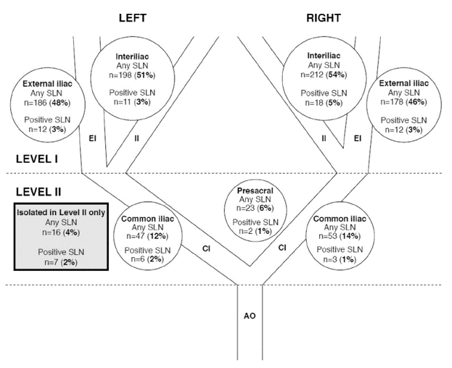
Nodal detection
rate
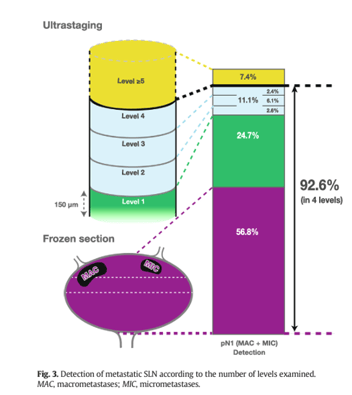
Ultrastaging